SAGL Crop Protection Division
I Have a New Product That Needs Testing!
What Now?
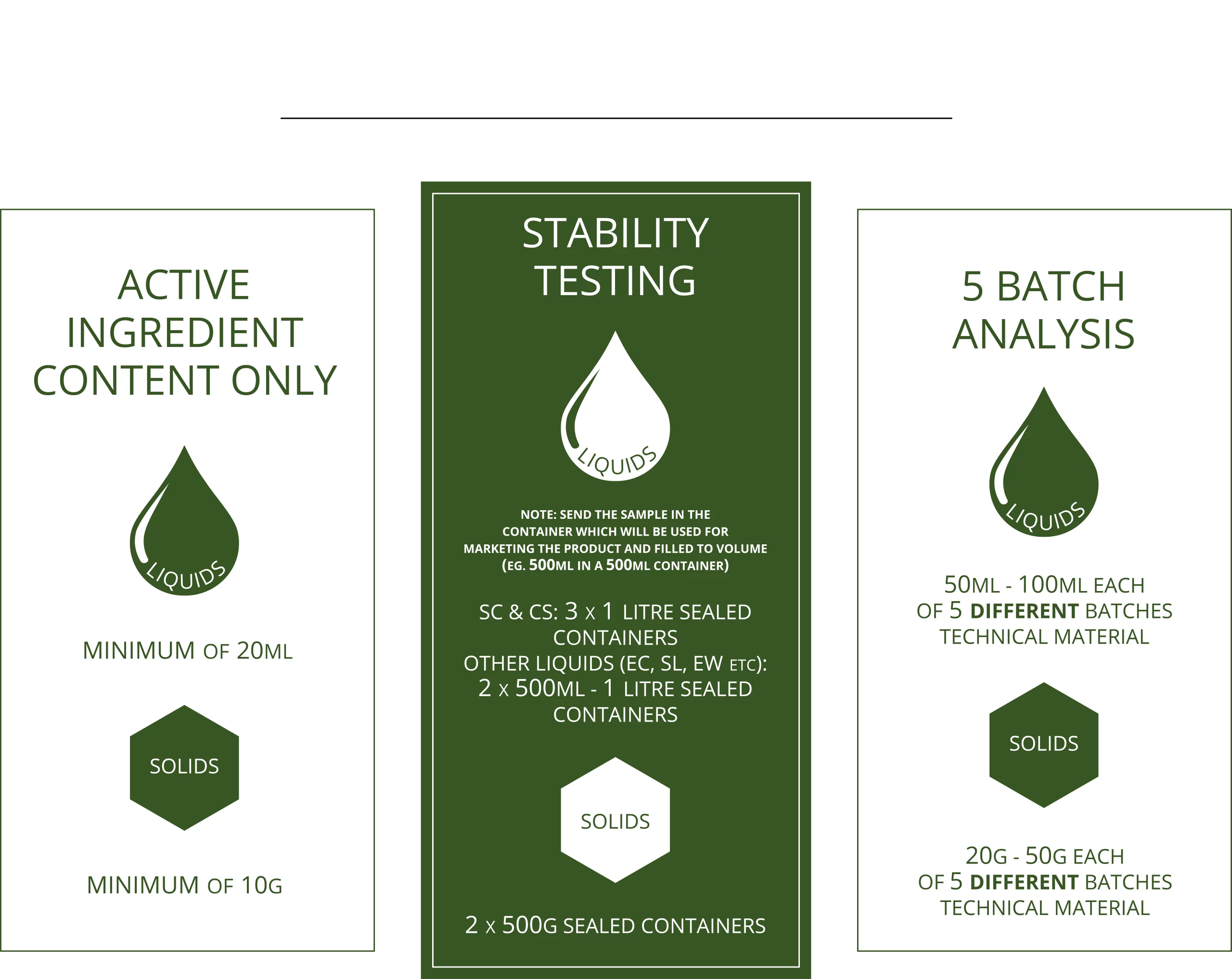
How Much Sample Do We Need?
5-Batch Analysis Sequential Process
- Five or more batches of technical material, from a specific manufacturer, are analysed to determine the impurity profile and to assess the purity of the active ingredient.
- All relevant impurities (impurities of toxicological significance) are quantified to ensure compliance with international specifications.
This work is usually conducted according to OECD GLP (OECD Series on Principles of Good Laboratory Practice (GLP) and Compliance Monitoring), a detailed OECD GLP Study Flow Chart is available on request which explains the process in detail.
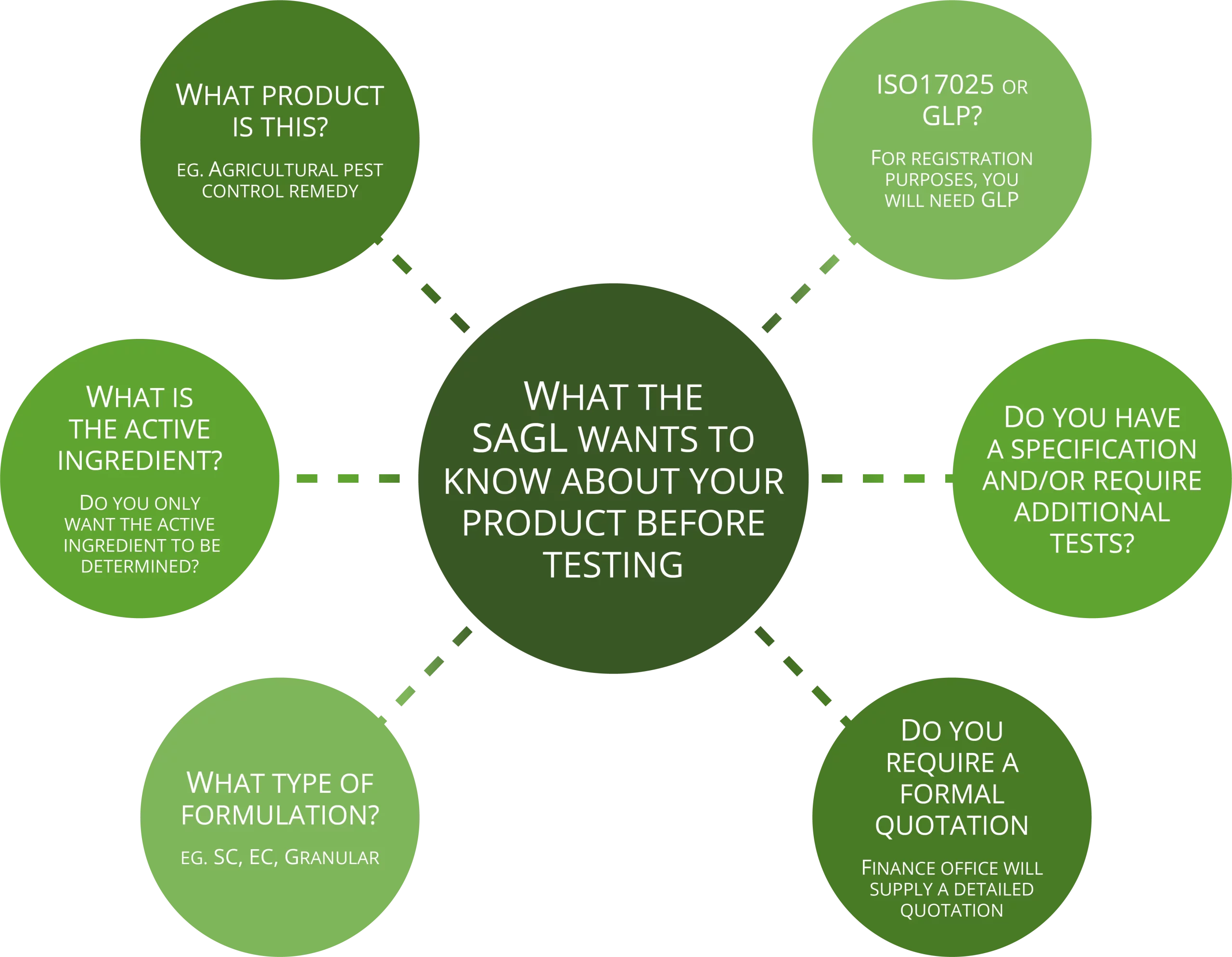
Initial Questions needed for a quotation:
What is the active ingredient?
What type of formulation (eg. SC, EC, granular etc.)
Will it be according to FAO Specifications? If not, furnish us with the Specification.
Name(s) of the relevant impurities
Extra tests required by the sponsor not stated by the specification (eg. Water content, viscosity, flash point etc.)
Note: We offer a screening analysis prior to the GLP analysis, to see whether the active ingredient content is on spec. (at little extra cost)
Semi-quantitative screening by GC-MS, GC-FID and or HPLC-UV(DAD) to determine all the compounds present above a level of 0.1 % w/w
Identification and characterisation of al significant impurities (impurities above 0.1 % w/w)
Developing analytical methods for the quantification of the active ingredient content, relevant impurities and any significant impurities.
Method validation and quantification of the active ingredient content, relevant impurities and significant impurities according to international requirements.
Method validation and quantification of the relevant impurities and significant impurities according to international requirements.
Determination of other physical properties e.g. pH, loss on drying , water content using Karl-Fisher titrator and others like acetone insoluble matter employing CIPAC methods
Generation and issuing of a full GLP compliant report or ISO-17025 accredited report with full client consultation, for regulatory submission.
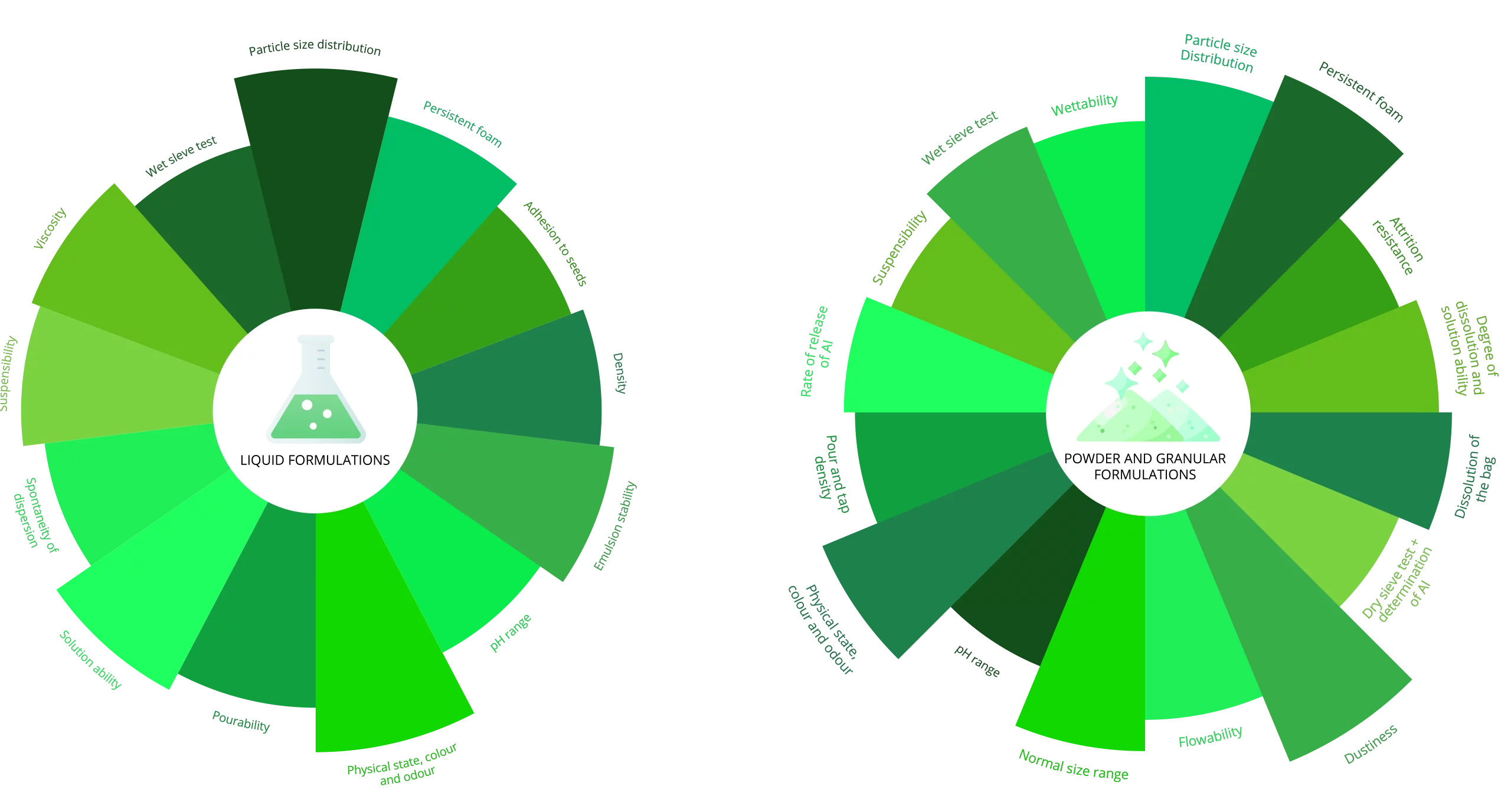
Physical & Chemical Studies on Formulated Plant Protection Products
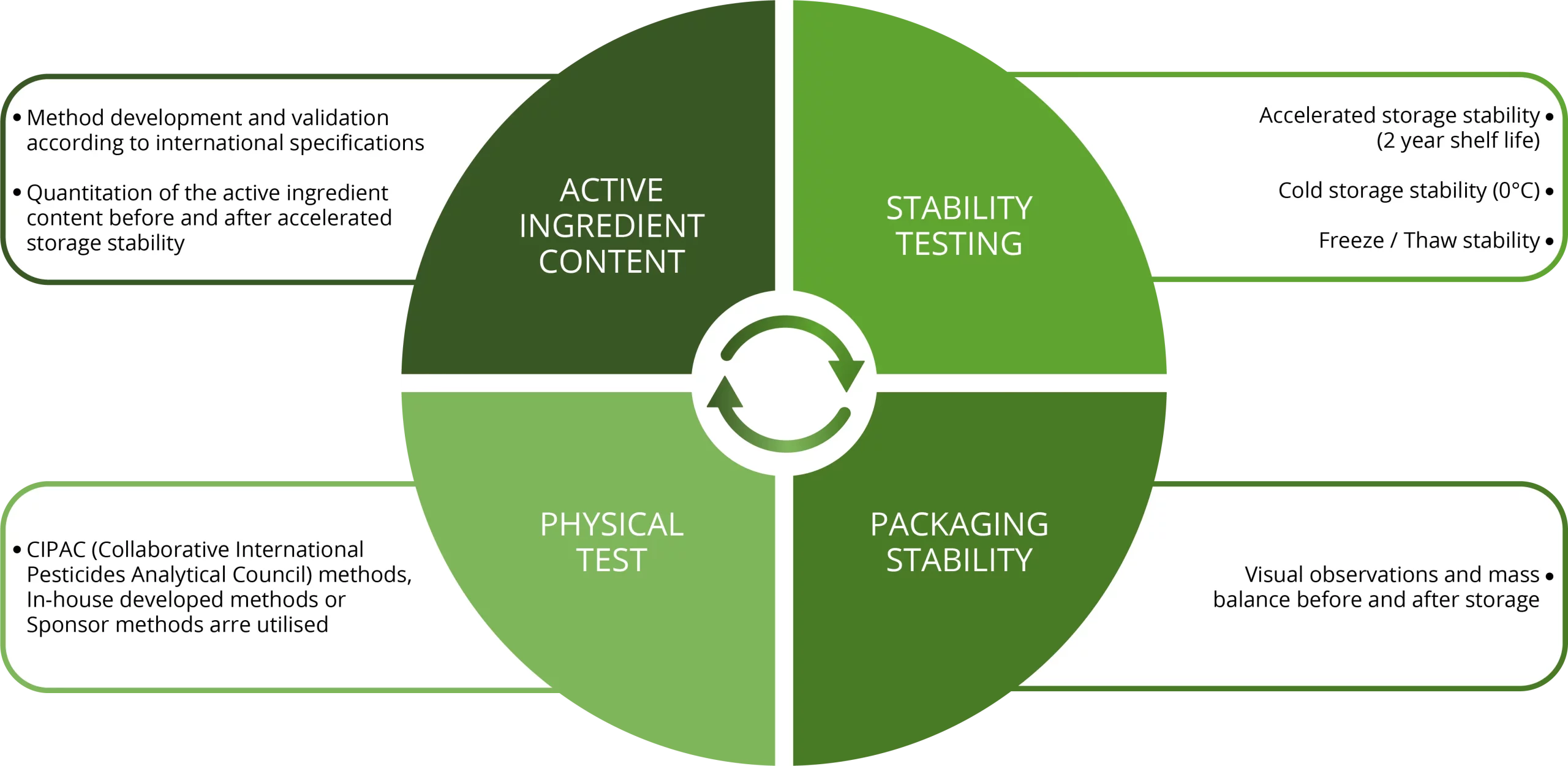
Physical and chemical studies on the formulated product is a regulatory requirement in the Agro-chemical Industry.
After storage various test are preformed on the product to access the stability of the product and to determine the shelf life of the product.
The following guidelines are followed:
- FAO (Food and Agricultural
- Organization of the United Nations)
- WHO (World Health Organization)
- APVMA (Australian Pesticides and Veterinary Medicines Authority)
- Any other regulatory requirement as specified by the sponsor or registration authority.
- Our team of experts are familiar with international requirements.
These studies can be performed with or without OECD GLP compliance.
Studies can also be conducted under ISO-17025 accreditation – (Active ingredient content, density, and pH determinations forms part of ISO17025 flexible scope of accreditation).
During these studies CIPAC (Collaborative International Pesticides Analytical Council) methods, In-house developed methods or methods received form our Sponsors are utilized.
Sponsors may also request any of the parameters below for quality control purposes.
Physical & Chemical Tests